You are here : AccueilAccueilActivités de recherche
- PDF version
-
Translations
Translational Research in Functional Imaging, Radiopharmaceuticals and Theranostic Biomarkers
The studies conducted by team 2 concerns research combining radiopharmaceutical approaches and genomic, transcriptomic and protein biomarker approaches in triple negative breast cancer (TNBC). These objectives include the development of companion radiotracers allowing an integrated approach to identify prognostic biomarkers. A structure dedicated to the clinical transfer of radiopharmaceuticals, CIRMEN, is highly implicated in the researches conducted by clinical investigators and open many perspectives in theranostic strategies
Concerning TNBCs, our group has already identified several biomarkers predictive of response or resistance to treatment, as well as rapid and aggressive relapse. One of the projects will be to define, by joint analysis of the transcriptome and somatic mutations, the molecular signatures of tumor clones that have or have not led to the appearance of metastases. Our group is also interested in the tumor immune microenvironment with identification of possible diagnostic, prognostic and predictive biomarkers following the analysis of benign cells surrounding tumor cells. We are also carrying out upstream work on in vitro and in vivo preclinical models. Our approach aims to (i) increase the sensitivity of tumor cells by maintaining anticancer drugs in cancer cells to (ii) bypass or attenuate the chemoresistance mechanisms, introduced by drug efflux pumps, called Multidrug Resistance (MDR) proteins. We have also developed three-dimensional (3D) cell culture models from different TNBC cancer lines with different tumor profiles representative of the pathology in vivo. (Dubois et al., 2017).
One of the original features of our joint research unit is the collaborative development of biomarkers and companion radiotracers. We are currently carrying out preclinical studies in collaboration with the Caminov company, on new radioligands of the nanobody (NB) type, radiolabeled by 68Ga, 18F or 111In targeting the Human Epidermal growth factor Receptor 3 (HER3). This type of molecular imaging will have the advantage of providing an exhaustive mapping of HER3 expression, ultimately allowing an optimal selection of patients who are candidates for therapy specifically targeting HER3. In parallel, molecular imaging of apoptosis is being developed with the aim of measuring the therapeutic response at a very early stage in TNBC patients treated with neoadjuvant chemotherapy.
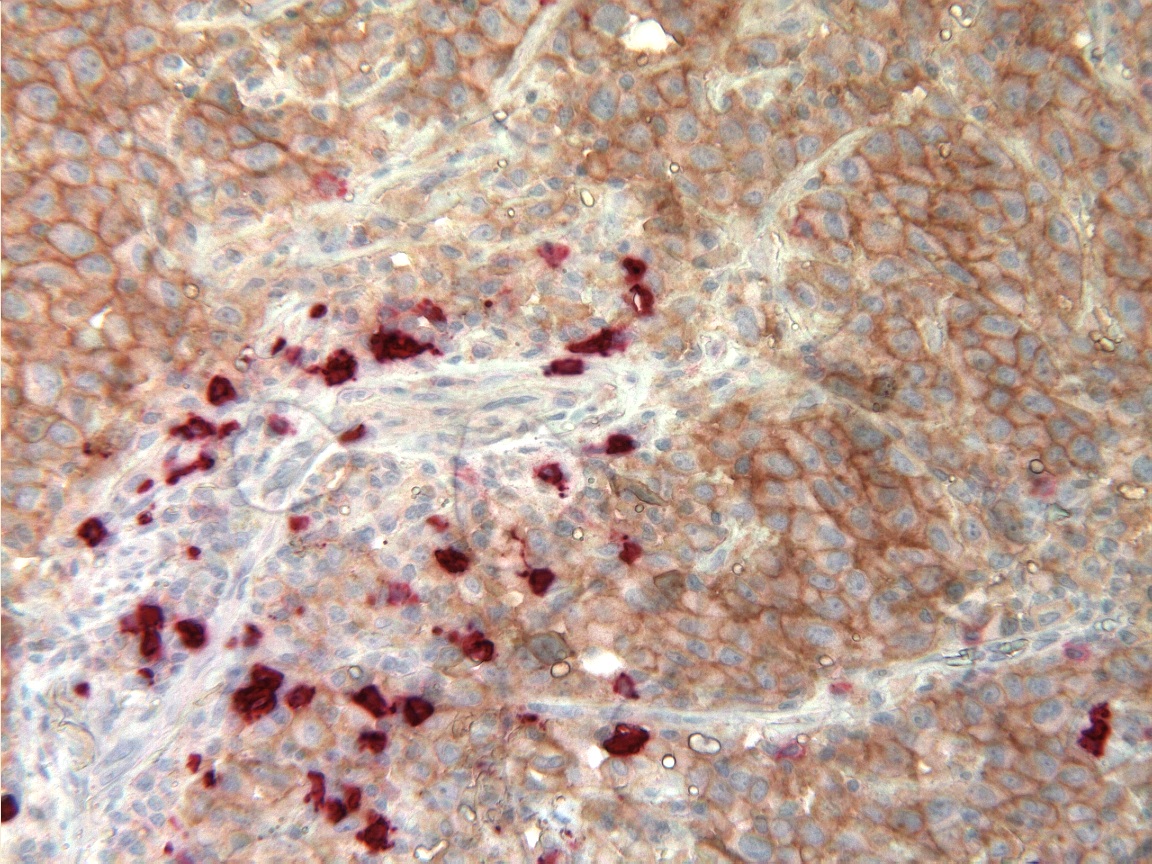
Other particularly original work in our unit concerns the evaluation of alterations caused by cancer and its treatment on reproductive functions in order to develop innovative theranostic means for fertility preservation.





 ENT
ENT Annuaire
Annuaire Se connecter
Se connecter